Quality Policy
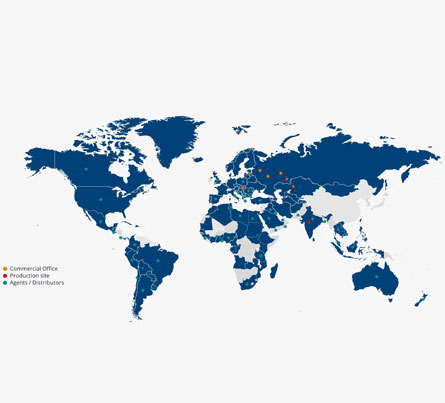
At Ultra Laboratories Pvt LTD, we believe that customer satisfaction, in terms of quality & commitment is our first and foremost responsibility.
Know moreNew Products
Over the years, Ultra Laboratories Pvt LTD has been working diligently to add every component of the Pharma value chain in its fold to be able to maintain consistent growth.
Know more